Research
Enzymes as Practical Catalysts for Organic Synthesis
Enzymes are intrinsically chiral catalysts since they are built from optically pure monomers (L-amino acids). This property makes them excellent starting points for developing methodology for stereoselective organic synthesis. In addition, enzymes are completely biodegradable and often work well in water, which are important properties when considering how chemical synthesis can be made more sustainable. Our past efforts have involved work on flavin-dependent Baeyer-Villiger monooxygenases, alcohol dehydrogenases and alkene reductases (also called ene-reductases or enoate reductases).
Pyridoxal-phosphate dependent enzymes. Pyridoxal phosphate (PLP) is a versatile cofactor involved in decarboxylations, transaminations, racemizations and C–C bond forming reactions. 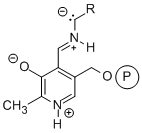 All the reactions involve Schiff’s base formation between an amino group on the substrate and the cofactor, and this stabilizes an anion on the carbon adjacent to the amine. We are currently exploring several PLP-dependent enzymes in an effort to make them even more useful for chemical synthesis.
All the reactions involve Schiff’s base formation between an amino group on the substrate and the cofactor, and this stabilizes an anion on the carbon adjacent to the amine. We are currently exploring several PLP-dependent enzymes in an effort to make them even more useful for chemical synthesis.
Non-heme iron oxygenases. Carotenoid cleavage oxygenases (CCOs, also known as CCDs) perform the biochemical equivalent of ozonolysis. These enzymes contain one tightly bound Fe ion in the active site and require no additional cofactors. Because they do not use ozone, ![]() these enzymes are much more environmentally benign and avoid toxicity and safety concerns. Past experience has shown that these enzymes accept an array of carotenoids; our goal is to broaden the substrate specificity to include alkenes of synthetic interest and add these to the synthetic toolkit.
these enzymes are much more environmentally benign and avoid toxicity and safety concerns. Past experience has shown that these enzymes accept an array of carotenoids; our goal is to broaden the substrate specificity to include alkenes of synthetic interest and add these to the synthetic toolkit.
Alkene reductases. We still maintain an interest in alkene reductases, particularly those of the Old Yellow Enzyme (OYE) family. We have carried out extensive protein engineering and x-ray crystallographic studies of these enzymes to make them useful for organic synthesis.
We gratefully acknowledge the National Science Foundation (CHE-1111791) for financially supporting our current work in biocatalysis.
Understanding and Improving Starch Biosynthesis in Corn
Increasing the starch content of corn – particularly at higher growth temperatures – will have an enormous impact on agriculture’s ability to meet the world’s food and non-food needs for this key raw material. Starch is a branched glucose polymer accumulated at high levels by the endosperm portion of cereal grains such as corn. It is biosynthesized from photosynthetically-produced carbohydrates in a pathway involving many different enzymes. In addition to understanding the biochemistry of key enzymes in the starch biosynthesis pathway, we also seek to engineer these enzymes for greater activity and resistance to thermal unfolding (denaturation) to enhance starch yield when corn is grown in high-temperature climates. This is a USDA-funded collaboration with groups at the University of Florida (Professors L. Curtis Hannah, Karen Koch, Donald McCarty and A. Mark Settles; Program in Plant Molecular and Cell Biology), Iowa State University (Drs. Alan Meyers and Tracie Hennen-Bierwagen) and the University of Wisconsin (Professor Bill Tracy)
 Increasing enzyme thermal tolerance to increase yield. It has been observed that starch yield in maize declines as the growth temperature increases. One reason is that the enzymes responsible for starch biosynthesis have limited stability toward heat-induced unfolding (denaturation). Results from transgenic corn have demonstrated a correlation between higher starch yield and and increased resistance of cytosolic ADP-glucose pyrophosphorylase to thermal unfolding. While the molecular basis for this observation is still under investigation, it suggests that additional yield increases might be possible if the thermal stabilities of other starch biosynthetic enzymes were also increased. We are part of a collaborative effort to identify the best protein targets and then apply protein engineering methods to enhance their thermal tolerances. This involves a multi-pronged search involving genetic, biochemical and bioinformatics strategies to uncover all enzymes important in starch biosynthesis, then direct protein studies to assess their thermal stabilities. Those found lacking will be subjected to improvement by iterative saturation mutagenesis strategies. Finally, the best variants will be placed into corn plants and tested under field conditions.
Increasing enzyme thermal tolerance to increase yield. It has been observed that starch yield in maize declines as the growth temperature increases. One reason is that the enzymes responsible for starch biosynthesis have limited stability toward heat-induced unfolding (denaturation). Results from transgenic corn have demonstrated a correlation between higher starch yield and and increased resistance of cytosolic ADP-glucose pyrophosphorylase to thermal unfolding. While the molecular basis for this observation is still under investigation, it suggests that additional yield increases might be possible if the thermal stabilities of other starch biosynthetic enzymes were also increased. We are part of a collaborative effort to identify the best protein targets and then apply protein engineering methods to enhance their thermal tolerances. This involves a multi-pronged search involving genetic, biochemical and bioinformatics strategies to uncover all enzymes important in starch biosynthesis, then direct protein studies to assess their thermal stabilities. Those found lacking will be subjected to improvement by iterative saturation mutagenesis strategies. Finally, the best variants will be placed into corn plants and tested under field conditions.
Metabolomics. Our team is also carrying out s systematic study of the changes in maize provoked by growth at high temperatures. This involves studies of changes in RNA, enzyme activities and small molecule metabolites. The Stewart group is leading the work in the latter area, working closely with the Southeast Center for Integrated Metabolomics and their partners.
We gratefully acknowledge the U.S. Department of Agriculture (2011-67003-30215) for financially supporting our current work in starch biosynthesis.


